Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Watanabe, So; Senzaki, Tatsuya; Shibata, Atsuhiro; Nomura, Kazunori; Takeuchi, Masayuki; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Horiuchi, Yusuke*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 322(3), p.1273 - 1277, 2019/12
Times Cited Count:4 Percentile:31.89(Chemistry, Analytical) -P adsorbent on extraction chromatography process
-P adsorbent on extraction chromatography processWatanabe, So; Sano, Yuichi; Sanda, Shuhei*; Sakurai, Shota*; Arai, Tsuyoshi*
Nihon Ion Kokan Gakkai-Shi, 30(1), p.8 - 16, 2019/01
Sano, Yuichi; Watanabe, So; Matsuura, Haruaki*; Nagoshi, Kohei*; Arai, Tsuyoshi*
Journal of Nuclear Science and Technology, 54(10), p.1058 - 1064, 2017/10
Times Cited Count:5 Percentile:37.06(Nuclear Science & Technology)For effective recovery of radioactive elements by adsorbents using polymer-immobilized silica (SiO -P) supports, the microstructure of SiO
-P) supports, the microstructure of SiO -P particles impregnated with CMPO as extractants and their change with the crosslinking degree of polymer (CDP) were investigated using STXM and EXAFS analyses; further, their relation with adsorption/elution behavior was discussed. The adsorption/elution tests using adsorbents with a different CDP demonstrated that a higher CDP inhibited the elution of adsorbed metal ions from the adsorbent. The results of STXM and EXAFS analyses suggested that adsorption by CMPO proceeds through the entire area in the adsorbent and the local structure around adsorbed metal ions is similar irrespective of the CDP. Conversely, STXM analyses implied the capture of eluents such as H
-P particles impregnated with CMPO as extractants and their change with the crosslinking degree of polymer (CDP) were investigated using STXM and EXAFS analyses; further, their relation with adsorption/elution behavior was discussed. The adsorption/elution tests using adsorbents with a different CDP demonstrated that a higher CDP inhibited the elution of adsorbed metal ions from the adsorbent. The results of STXM and EXAFS analyses suggested that adsorption by CMPO proceeds through the entire area in the adsorbent and the local structure around adsorbed metal ions is similar irrespective of the CDP. Conversely, STXM analyses implied the capture of eluents such as H O by polymers with high CDP, which suppresses the prompt elution of adsorbed metal ions from the adsorbent.
O by polymers with high CDP, which suppresses the prompt elution of adsorbed metal ions from the adsorbent.
Watanabe, So; Nomura, Kazunori; Kitawaki, Shinichi; Shibata, Atsuhiro; Kofuji, Hirohide; Sano, Yuichi; Takeuchi, Masayuki
Procedia Chemistry, 21, p.101 - 108, 2016/12
Times Cited Count:14 Percentile:99.14(Chemistry, Inorganic & Nuclear)Naganawa, Hirochika; Suzuki, Hideya*; Noro, Junji*; Kimura, Takaumi
Chemical Communications, (23), p.2963 - 2965, 2005/06
A "superweak" anion, TFPB-, gives rise to a field effect on the selectivity for Am over Ln
over Ln in their extraction from aqueous HNO
in their extraction from aqueous HNO solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
Zhang, P.*; Kimura, Takaumi; Yoshida, Zenko
Solvent Extraction and Ion Exchange, 22(6), p.933 - 945, 2004/00
Times Cited Count:13 Percentile:45.29(Chemistry, Multidisciplinary)no abstracts in English
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.874 - 877, 2002/11
To facilitate the management of high-level liquid waste (HLLW) and minimize its long-term radiological risk in geologic disposal, we have proposed an advanced partitioning process by extraction chromatography using a minimal organic solvent and compact equipment to separate long-lived minor actinides (MA) and specific fission products (FP) such as Zr and Mo from nitrate acidic HLLW solution. Novel silica-based extraction-resin for elemental groups separation was prepared by impregnating CMPO (octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide) into a macro-reticular styrene-divinylbenzene copolymer immobilized in porous silica particles with a diameter of 50  m (SiO
m (SiO -P). Separation experiments for simulated HLLW solutions containing a trace amount of
-P). Separation experiments for simulated HLLW solutions containing a trace amount of  Am (III) and macro amounts of typical FP elements were carried out by column chromatography. It was found that the elements in the simulated HLLW were successfully separated to the following three groups: Cs-Sr-Rh-Ru, Pd-Ln-Am and Zr-Mo.
Am (III) and macro amounts of typical FP elements were carried out by column chromatography. It was found that the elements in the simulated HLLW were successfully separated to the following three groups: Cs-Sr-Rh-Ru, Pd-Ln-Am and Zr-Mo.
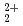 across the interface between aqueous and nitrobenzene solutions in the presence of Octyl(phenyl)-N,N-diisobutylcarbamoyl-methyl-phosphine oxide (CMPO)
across the interface between aqueous and nitrobenzene solutions in the presence of Octyl(phenyl)-N,N-diisobutylcarbamoyl-methyl-phosphine oxide (CMPO)Ying, M.*; Kitatsuji, Yoshihiro; Kimura, Takaumi; Yoshida, Zenko
Journal of Nuclear and Radiochemical Sciences, 2(1-2), p.11 - 15, 2001/12
no abstracts in English
Naganawa, Hirochika; Suzuki, Hideya; Tachimori, Shoichi; Nasu, Akinobu*; Sekine, Tatsuya*
Physical Chemistry Chemical Physics, 3(12), p.2509 - 2517, 2001/06
Times Cited Count:34 Percentile:70.77(Chemistry, Physical)no abstracts in English
; ;
JNC TN8400 2001-022, 60 Pages, 2001/03
A numerical simulation code for the TRUEX (Transuranium Extraction) process was developed. Concentration profiles of americium and europium were calculated for some experiments of the counter current extraction system those were carried out in CPF (Chemical Processing Facility) by using the code. Calculation profiles were in agreement with the experimental results. Operational conditions were also examinted for the americium recovery experiment by the TRUEX process carried out in the Plutonium Fuel Center. It was shown that lowering the concentration of nitric acid in the scrub solution and decreasing the flow rate of solvent and strip solution was effective for improving the performance of the stripping step and reducing the volume of the waste solution. In order to find the optimum conditions for various experiments, this simulation code was modified to calculate the concentration profiles of other metal elements such as zirconium and iron and the effect of oxalic acid on the extraction behavior of the metal elements. The calculated concentration profiles of americium and europium were varied by this modification. In the experiment at CPF, the calculations were carried out to obtain recovery ratio of americium in the product stream with the amount of oxalic acid added to the process. This calculation result showed that it was possible to improve the performance of decontamination of fission products by increasing oxalic acid concentration added to the process. The calculation was also carried out for finding the optimum conditions of oxalic acid concentration added to the europium recovery process.
Wahyuni, S.*; Hirota, Koichi; Hakoda, Teruyuki; Arai, Hidehiko; Hashimoto, Shoji; Kawamoto, Fumio*; Mukunoki, Yasuo*
Bulletin of the Chemical Society of Japan, 73(8), p.1939 - 1943, 2000/08
Times Cited Count:2 Percentile:16.96(Chemistry, Multidisciplinary)no abstracts in English
Yaita, Tsuyoshi
Proceedings of International Youth Nuclear Congress 2000 (CD-ROM), 4 Pages, 2000/00
no abstracts in English
Suzuki, Hideya*; ; Tachimori, Shoichi
JAERI-Research 98-050, 48 Pages, 1998/09
no abstracts in English
Sano, Yuichi; *; ; *; Koyama, Tomozo; Tanaka, Yasumasa
PNC TN8410 96-362, 19 Pages, 1996/10
The coordination properties of the lanthanide (La, Ce, Pr, Nd, Sm and Eu) complexes in lanthanide/TBP (tributylphosphate), lanthanide/CMPO (octyl(phenyl)-N,N-diisobutycarbamoylmethylphosphine oxide) and lanthanide/CMPO/TBP systems were investigated by NMR (nuclear magnetic resonance) measurements. In the lanthanide/CMPO/TBP system, it is shown that the structure of lanthanide complex is changed by the concentration ratio for added CMPO to the lanthanide ion ; lanthanide/CMPO/TBP system ([CMPO]/[Ln]  3 (mole ratio)) NO
3 (mole ratio)) NO - the similar coordination in the Ln/TBP system TBP, CMPO - These coordinate to the lanthanide ion together. (the existence of several complexes) lanthanide/CMPO/TBP system ([CMPO]/[Ln}
- the similar coordination in the Ln/TBP system TBP, CMPO - These coordinate to the lanthanide ion together. (the existence of several complexes) lanthanide/CMPO/TBP system ([CMPO]/[Ln}  3(mole ratio)) NO
3(mole ratio)) NO - the same coordination in the Ln/CMPO system TBP, CMPO - Only CMPO coordinates to the lanthanide ion. TBP doesn't exist within the first coordination sphere, but affects the CMPO exchange reaction.
- the same coordination in the Ln/CMPO system TBP, CMPO - Only CMPO coordinates to the lanthanide ion. TBP doesn't exist within the first coordination sphere, but affects the CMPO exchange reaction.
Shibata, Atsuhiro; P.Y.COR*; *; ; ; *; Tanaka, Yasumasa
PNC TN8410 95-112, 100 Pages, 1995/05
PNC has developed the TRUEX process to recover transuranium elements from high level liquid waste. This process uses CMPO as extracting solvent. DIAMEX process which uses Diamide has been developed bY CEA in France. The aim of basic studies was to get some comparison data between CMPO and Diamide. We have conducted the experiments regarding properties of Ln(III) extraction and behavior of third phase formation. Also, we have investigated properties of Ln(III) extraction with TBP and DBBP to compare bidentate ligand with monodentate ligand. This study was conducted as a frame of PNC/CEA collaboration on minor Actinide partitioning. The conclusions drawn from our studies can be summarized as follows; (1)Trivalent Lanthanides extraction reaction. CMPO, TBP and DBBP ; Ln + 3NO
+ 3NO + 3Extractant
+ 3Extractant 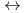 Ln(NO
Ln(NO )
)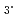 3Extractant. Diamide; Ln
3Extractant. Diamide; Ln + 3NO
+ 3NO + m Diamide
+ m Diamide 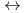 Ln(NO
Ln(NO )
)
 m Diamide : m=O.7
m Diamide : m=O.7 1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO
1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO  Diamide
Diamide  DBBP
DBBP  TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2
TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2  10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
; ; ; ; *
PNC TN8410 93-046, 46 Pages, 1993/03
The TRUEX process has been developed to recover transuranium elements from high level liquid waste. This process uses octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO) which is able to extract tri-valent actinides. It is already well known that a third phase appears at the interface when rare earths and other metal ions are extracted from a concentrated solution into TRUEX solvent which consists of CMPO with TBP - dodecane mixture. The basic researches concerning behavior of third phase formation and elimination were done in previous experiments. Succseively, dependency of temperature on organic phase composition, behavior of U extraction, applicability to high level liquid waste and diluent effect were investigated in these experiments. Consequently, these results were made obviously. By composition analysis of solvent which extracted nitric acid, it was found that not only concentration of extracted species, but temperature also influences on the composition of split phase. Extraction of high uranium concentration caused yellow precipitation. Critical concentration for precipitation were constant in each system and not dependent on temperature significantly. This precipitation was considered to affect extraction procedure, so that the condition should be selected carefully. In experiment using simulated waste, it's found that with appropriate condition of TBP concentration and temperature, dilution of concentrated liquid waste would not be necessary. From this work, 1.4 M for TBP concentration and 40  C are promisible and that are typical values for concentrated waste. However, to determine the practical conditon for real waste treatment, further experiments should be done. From comparison of hydrocarbon composed of 12 carbons with n-dodecane as diluent, carbon chain length and banching influence on critical concentration of third phase formation.
C are promisible and that are typical values for concentrated waste. However, to determine the practical conditon for real waste treatment, further experiments should be done. From comparison of hydrocarbon composed of 12 carbons with n-dodecane as diluent, carbon chain length and banching influence on critical concentration of third phase formation.